Abstract
Introduction
The Blood and Marrow Transplant Clinical Trials Network study (BMT CTN 1102, NCT02016781) was a multicenter, biologic assignment trial in older adults aged 50-75 with higher risk de novo MDS (IPSS Intermediate-2 or High) who were candidates for reduced-intensity conditioning (RIC) allogeneic HCT. The trial compared outcomes of those with a suitable HLA-identical sibling or unrelated donor (Donor arm) to those where no donor was identified (No Donor arm) within a search window of 90 days. The trial reported a survival benefit for patients in the Donor arm compared to the No Donor arm [Nakamura et al, JCO 2021, in press]. Here, we compare the health-related quality of life (QOL) for patients between the two arms through 36 months after enrollment. We also describe the predictors and trajectories of QOL.
Methods
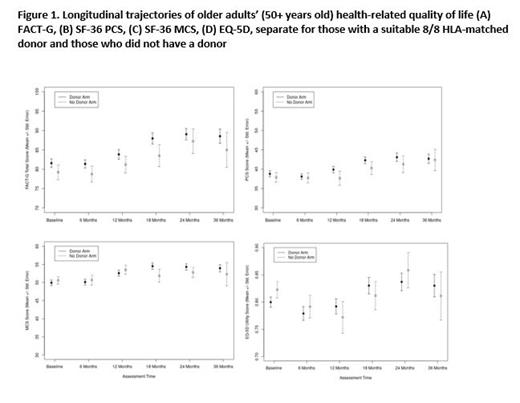
English and Spanish-speaking subjects were invited to complete patient-reported outcome (PRO) measures, including the Functional Assessment of Cancer Therapy - General (FACT-G), the SF-36 yielding a Physical Component Score (PCS) and Mental Component Score (MCS), and the EQ-5D, at enrollment and every 6 months until 24 months, then 36 months after enrollment. Validation studies indicate a clinically meaningful difference of 5 points for FACT-G, PCS, and MCS and 2 points for associated subscales. To account for the missingness of assessments, including those missing due to death, we compared each score between arms using an inverse probability weighted - independent estimating equation (IPW-IEE) model, which models the scores in surviving patients while using IPW to account for baseline variables and follow-up outcomes that impact the likelihood of missingness. Since 4 scores were evaluated, a Bonferroni corrected significance level of 1.25% = 5% / 4 was used. Cox regression models were used to evaluate the impact of QOL measures on overall and leukemia free survival. The IPW-IEE models adjust for baseline score and follow-up assessment time as well as age, race/ethnicity, performance status, IPSS score, duration of disease, and response to prior hypomethylating therapy; the Cox models adjusted for the 6 latter variables and treatment arm. Trajectories of QOL are shown by plotting mean +/- standard error by group over time.
Results
Between January 2014 and November 2018, 384 subjects (median age 66.7 years, range: 50.1-75.3) were enrolled at 34 centers and biologically assigned to Donor (n=261) or No Donor arm (n=123) by 90 days from enrollment. For the Donor arm the median duration from registration to HCT was 3.9 months (range 0.3-20.7). Completion rates were generally high at 65-78% of eligible survivors at each timepoint. At enrollment, 204 subjects (78.2%) in the Donor arm and 85 subjects (69.1%) in the No Donor arm completed at least one QOL form, with 4 patients unable to complete due to language (non-English or Spanish speaking). While there were some small differences at 18 months favoring the Donor arm, no clinically significant differences in PRO scores or subscales were seen between the arms at any timepoint (Figure 1) or in the scores over time. In general, similar trajectories for the Donor arm were seen for each PRO, with most decreasing or stable from baseline to 6 months and improving thereafter. Compared to published averages of U.S cancer populations, FACT-G means in both arms were higher beginning at 18 months. Baseline and 6-month PRO scores were the strongest predictors of later PRO scores despite adjusting for patient demographic and clinical factors. Overall survival was predicted by baseline FACT-G <70 (HR=1.61, p<.01) and PCS scores <40 (HR=1.82, p<0.001), while leukemia-free survival was predicted by baseline FACT-G <70 (HR=1.61, p<0.01) and PCS <40 (HR=1.80, p<0.002). No associations of PROs with AML transformation, relapse, or acute or chronic GVHD were found. Non-compliance with biologic assignment was less likely in Donor arm compared to No Donor, but baseline QOL was not a confounding factor.
Conclusion
In older adults with MDS, the survival advantage associated with Donor availability and HCT does not come at the cost of worse QOL in comparison to the No Donor arm. Baseline PRO scores were the strongest independent predictors of subsequent QOL outcomes and survival, even after controlling for clinical and patient-level factors. These results should reassure older patients and clinicians who prefer curative approaches to MDS.
Cutler: Mallinckrodt: Consultancy; Editas: Consultancy; CareDx: Consultancy; Kadmon: Consultancy; Pfizer: Consultancy; Incyte: Consultancy; Cimeio: Consultancy; Deciphera: Consultancy; Omeros: Consultancy; Syndax: Consultancy; Mesoblast: Consultancy; Jazz: Consultancy. Saber: Govt. COI: Other. Lee: Takeda: Research Funding; Syndax: Research Funding; Pfizer: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Kadmon: Research Funding; National Marrow Donor Program: Membership on an entity's Board of Directors or advisory committees; Janssen: Other; Incyte: Research Funding; AstraZeneca: Research Funding; Amgen: Research Funding. Shaw: mallinkrodt: Other: payments; Orca bio: Consultancy. Horowitz: Magenta: Consultancy, Research Funding; Astellas: Research Funding; Jazz Pharmaceuticals: Research Funding; GlaxoSmithKline: Research Funding; Genentech: Research Funding; CSL Behring: Research Funding; Gamida Cell: Research Funding; Allovir: Consultancy; Daiicho Sankyo: Research Funding; Amgen: Research Funding; bluebird bio: Research Funding; Chimerix: Research Funding; Actinium: Research Funding; Medac: Research Funding; Kiadis: Research Funding; Xenikos: Research Funding; Vor Biopharma: Research Funding; Bristol-Myers Squibb: Research Funding; Regeneron: Research Funding; Pharmacyclics: Research Funding; Pfizer, Inc: Research Funding; Orca Biosystems: Research Funding; Omeros: Research Funding; Novartis: Research Funding; Miltenyi Biotech: Research Funding; Mesoblast: Research Funding; Kite/Gilead: Research Funding; Janssen: Research Funding; Sanofi: Research Funding; Seattle Genetics: Research Funding; Shire: Research Funding; Sobi: Research Funding; Stemcyte: Research Funding; Takeda: Research Funding; Tscan: Research Funding; Vertex: Research Funding.